Abstract
Background
Maintenance therapy (MT) deepens response and prolongs progression free survival (PFS) in patients with newly diagnosed multiple myeloma (NDMM) after frontline regimens. Ixazomib, a 2nd generation oral proteasome inhibitor (PI), has been approved for MT because of its convenience and tolerability.
Aims
We conducted this prospective multi-center study to compare the efficacy and safety of Ixazomib (I-MT) or Ixazomib plus Lenalidomide (IL-MT) to Lenalidomide (L-MT) as maintenance regimen in NDMM patients.
Methods
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital and registered (NCT04217967). NDMM patients were enrolled from 10 centers of North China MM Registry, since September 2019. After 4 cycles of front-line induction therapy, patients reached partial response (PR) would receive autologous stem cell transplantation (ASCT) if eligible, or receive up to 5 cycles of front regimens if ineligible, then start MT. Patients did not reach PR would switch to 2nd-line induction for 2-5 cycles and start MT once PR was achieved. 4mg of Ixazomib was given on day 1,8,15, and 25mg of Lenalidomide every other day on days 1-21 of 28day cycles. Patients in dual drug group were administrated with both Ixazomib and Lenalidomide, dose as listed above. The primary endpoint was PFS from MT.
Results
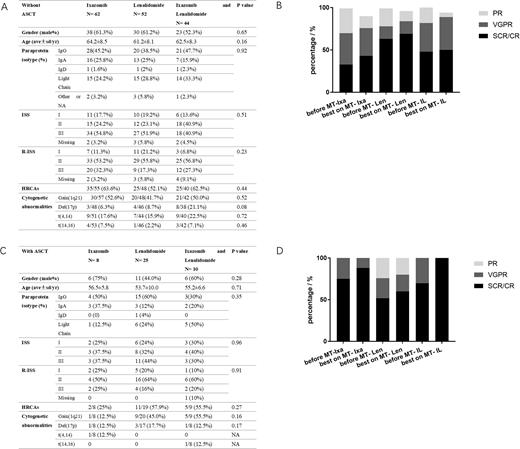
A total of 201 patients were enrolled, including 70 in I-MT, 77 in L-MT and 54 in IL-MT. The demographic and clinical characteristics, including gender ratio, age, paraprotein isotype, ISS, R-ISS, were comparable among different MT regimen groups receiving ASCT or not at baseline (Fig A). The proportions of patients with high-risk cytogenetic abnormalities (HRCAs), defined as amplification 1q21, deletion 17p, t(4,14) and t(14,16), were slightly lower in I-MT with ASCT. While the proportion of deletion 17p was higher in IL-MT without ASCT.
The median follow-up duration was 15.6, 16.4 and 14.6 months in I-MT, L-MT and IL-MT non-transplant patients, while 22.9, 18.9 and 14.3 months in ASCT group, respectively. Deepening of response depth was recorded in 16.1%, 7.7%, and 20.5% of the non-transplant patients, while 12.5%, 16%, and 30% of the patients in ASCT group, respectively. In non-transplant patients, the disease progression rate was 29.0%, 15.4% and 27.3%, whereas 25%, 24% and 20% in ASCT group. The median PFS and OS were not reached (NR) in all groups.
As for safety, the prevalence of peripheral neuropathy in whole cohort was 17.1% on I-MT, 7.8% on L-MT and 18.6% on IL-MT. The incidence of gastrointestinal events was 22.9%, 1.4% and 13.0%, respectively. Hematologic toxicities developed in 5.7%, 5.6% and 9.3% of the patients. Infection rates were 10%, 2.8% and 3.7%. 3 patients had withdrawn from MT due to adverse events, 2 for PN and 1 for hematologic toxicity.
Conclusions
Due to inadequate access to melphalan and low rate of ASCT in China, the PFS of NDMM was lower than those in western countries. We design this multi-centered prospective study to evaluate if dual drug MT will further strengthen response and make up the gap. Though the primary endpoint--PFS has not been reached in all treatment groups, dual drug MT improves response most and is quite tolerable.
Figure Legend:
A) Baseline characteristics of non-transplant patient on Ixazomib-MT, Lenalidomide-MT, Ixazomib and Lenalidomide-MT.
B) Changes of response depth after maintenance with different regimens in non-transplant patients.
C) Baseline characteristics of ASCT patient on Ixazomib-MT, Lenalidomide-MT, Ixazomib and Lenalidomide-MT.
D) Changes of response depth after maintenance with different regimens in patients with ASCT.
Abbreviations: ISS: international staging system; R-ISS: revised-ISS; HRCAs: high risk cytogenetic abnormalities. sCR: stringent complete remission. CR: complete remission. VGPR: very good partial remission. PR: partial remission. * p < 0.05.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.